Energy Levels¶
Energy Quantisation¶
Energy is quantised with Planck’s constant (\(h\)) as the proportionality factor
Energy Levels¶
Electronic¶
These the quantum electronic states of the molecule
Translational (3 DOF)¶
The molecule as a whole can move in space (as a single unit)
Rotational (linear = 2 DOF nonlinear = 3 DOF)¶
The molecule can rotate in space
Where \(I=\) The moment of inertia of the system (\(I=\sum_{j=1}^nm_j(x_j-x_{cm})^2\) for linear molecules) and the degeneracy of a given level is \(\mathrm{g}_J=2J+1\)
Momentum for nonlinear molecules
For nonlinear molecules, we need to consider the symmetry, as it will have multiple moments of inertia, based on how it rotates
So this looks like:
Vibrational (linear = 3n-5 DOF nonlinear = 3n-6 DOF)¶
Note
The subtraction from the degrees of freedom is removing the translational and rotational degrees of freedom. All molecules have a total of \(3n\) degrees of freedom
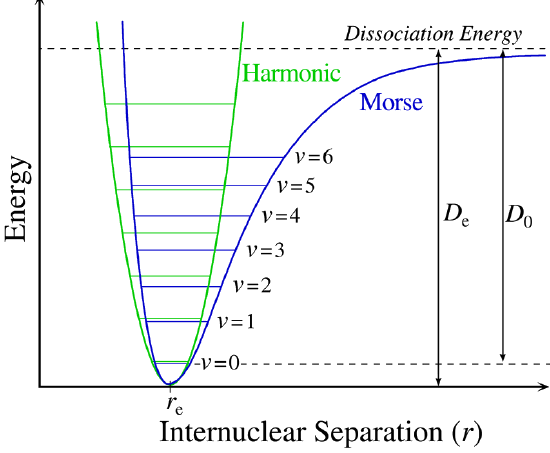
The regions bonds can vibrate with energy
This is a harmonic oscillator model and the degeneracy is \(\mathrm{g}_v=1\)
The space between each of the energy levels is \(=h\nu\), and the first energy level is separated from the depth of the well by the ZPVE \(\bigg(\frac{h\nu}{2}\bigg)\)
For the Morse potential, we can use the equation
The individual vibrations have different normal modes (types of vibration), and so the total vibrational energy is the sum of all the normal modes
Total energy¶
The total energy of a molecule can be described as the sum of all of these energies
Spacing of Energy Levels¶
These energy levels build upon each other, so for every electronic level is a series of vibrational levels and for every vibrational levels there are a series of rotational levels and for every rotational level there are a series of translational levels