Chemical Additives in Food¶
Chemical additives are governed by Food Standards Australia and New Zealand (FSANZ) a,d they regulate the maximum amounts of additives in food products, the allowable usage, and package labelling laws.
All food additives must be listed in in the ingredients label and they must be listed by their classs name, followed by the name of the additive or their food additive number.
- E.g. Colour (caramel I) or Colour (150a)
The exceptions to package labelling laws is for unpackaged items and for packages with a surface area of \(<100cm^2\). Enzymes and most flavour additives can be listed by their class name only and if an ingredient makes up less than \(5\%\) of the final product it doesn’t need to be listed.
The food additive classes are as follows:
| Number | Class Name |
|---|---|
| 100-199 | Colours |
| 200-299 | Preservatives |
| 300-399 | Antioxidants and acidity regulators |
| 400-499 | Thickeners, stabilisers and emulsifiers |
| 500-599 | pH regulators and anti-caking agents |
| 600-699 | Flavour enhancers |
| 700-799 | Antibiotics |
| 900-999 | Miscellaneous |
| 1100-1599 | Additional chemicals |
Generally Recognised as Safe (GRAS)¶
All food additives that are used should be GRAS, which is a classification that was implemented in 1958 by the US FDA to designate chemicals or substances that is considered safe for consumption by experts.
Definition - Food Additives
“Are chemicals added to foods for a particular reason during processing or storage, which could affect the characteristics of the food, or become part of the food”
Additives exclude food ingredients, such as:
- Salt, sugar, spices or seasonings
- Vitamins and minerals
- Veterinary drugs or agricultural chemicals
The main function of functions of food additives are to:
- Improve storage properties
- Improve or maintain nutritional value
- Increase healthfulness
- Make food more appealing (colours, anti-caking, thickeners, etc.)
- Improve processing and preparation
There are five categories of food additives that wlll be covered in this unit
Preservatives¶
Traditional Methods¶
The most fundamental and oldest reason for using additives is to preserve the quality of the food. Traditional methods include; salting, pickling, smoking, preserving in concentrates and drying.
While these methods don’t use chemical preservatives, they’re not inherently safe as they may not prevent all bacterial and fungal growth. these foods can still be potentially hazardous
Chemical Methods¶
While traditional methods are still often used, quite often chemical additives are also added to increase shelf life, in an increasingly convenient food market
Nitrates and Nitrites¶
Typically potassium based (\(\ce{KNO2/KNO3}\)), they are added to curing salts in the preservation of cured meats. The nitrates and nitrites react with myoglobin in the meat, giving them their distinct colour. This is largely thought to work due to the inhibition of growth of clostridium botulinum*
The biggest concern with them is that secondary amines in food may react to form highly carcinogenic nitrosamines (\(\ce{R2N-N=O}\)). The acceptable daily intake (ADI) of nitrates is \(60\:mg/day\), tough the nitrate/nitrite intake form natural sources is often higher than from processed foods (\(50\:mg/100g\) for processed mead, compared to \(200\:mg/100g\) of spinach)
Analysis is typically colorimetric and carried out using the Griess reagent. In the test, \(\ce{NO2-}\) and the Griess reagent are combined to form an azo dye that has a red/pink colour and form stoichiometrically to the concentration of nitrites.
Nitrates can either be measured using an ion-sensitive electrode, or can be reduced to nitrites for analysis with Griess reagent.
Sulfites¶
Sulphur dioxide (\(\ce{SO2}\)) and salts of sulphurous acid (\(\ce{H2SO3}\)) are used in wine, meat products, dried fruit and vegetables, as it acts as both an antioxidant and an antimicrobial agent. \(\ce{SO2}\) is a gas that can be pressurised and injected directly into liquids. it can also be dissolved as an ice cold aqueous solution as it’s sulphurous acid form (\(\ce{SO2 + H2O -> H2SO3}\))
Sulphites inhibit bacteria, but are less effective at inhibiting yeasts, making them useful for the fermentation of wine. In wine, specially bred \(\ce{SO2-}\) resistant yeast are widely used. Sulphites have variable antiseptic activity, and are more effective at lower pH. This is because the undissociated acid (\(\ce{H2SO3}\)) is significantly more effective than the dissociated acid (\(\ce{HSO3-}\)) and the low pH ensures that it remains protonated.
Fresh fruits can also be treated with \(\ce{SO2}\) to prevent the growth of surface mould.
It’s difficult for manufacturers to use excessive amounts of sulphites, as they can be tasted anywhere within the range of \(200-500\:ppm\). The ADI is set at \(1.5\:mg/kg/day\), however sulphates are an allergen, so the reduction of them is probably a good thing. In other foods, they can be effectively replaced by sorbic acid or ascorbic acid, in winemaking, due to their selectivity against yeast, they are non-replaceable.
Sulphites are volatile, as they’re dissolved gasses so the residual amounts in the sample will likely be less than what was initially applied. A colorimetric method is to add malachite green, as the \(\ce{SO2}\) will bleach is quantitatively. It’s also possible to distil off the \(\ce{SO2}\) ans \(\ce{H2SO4}\) and titrate it with a standardised base/
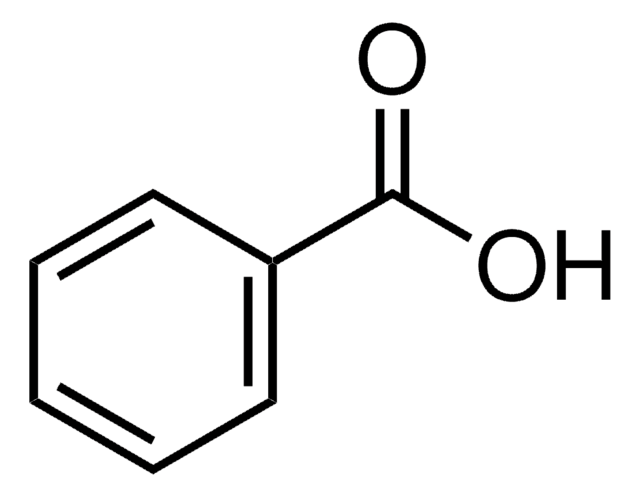
Benzoic acid¶
Preservative 201 - 212, is commonly used in soft drinks to prevent the growth of microorganisms in acidic foods. It has to be used in a low pH, as it’s only active in its protonated form (less than pH 4.5), however this makes it effective in such environments, which can be favourable for the flavour of food.
It’s commonly used in soft drinks, high acid food, fruit drinks, cider, pickles, margarines, salad dressings, soy sauce and jams and is naturally occurring in many types of berries, plums, prunes and some spices. In aqueous environments however the protonated form is less soluble, so adding it as the deprotonated conjugate base, sodium benzoate can increase the solubility.
The ADI is \(\sim0.35\:g/day\), however it is often found in conjunction with benzene, which is highly carcinogenic.
It’s typically identified using GC or HPLC.
Organic Chemicals¶
Sorbic (\(\ce{H3C-CH=CH-CH=CH-COOH}\)) and propionic acid (\(\ce{CH3CH2COOH}\)) (e200-203 and e280-283 respectively) are both effective ony in their protonated forms (pKa of 4.8 and 4.9 respectively). Sorbic acid has an ADI of \(\sim1.75\:g/day\) and PA has not ADI.
Sorbic acid and other sorbates are effective against fungi and are commonly used in wine, fruit juice, dried fruit, cottage cheese, meat and fish products
Analysis is typically performed by GC
Nisin¶
Nisin is an antimicrobial polypeptide, produced by some strains of l. lactis. it contains lots of disulphide linkages and is in a class known as bacteriocins. It’s been used to preserve processed cheese and since it contains no aromatic amino acids, and is heat stable, it can be used to extend the shelf life of sterilised milk.
Its one drawback is that it’s effectiveness decreases as the bacterial load increases though, so it can’t be used as a catch all preventative.
Anti-Oxidants¶
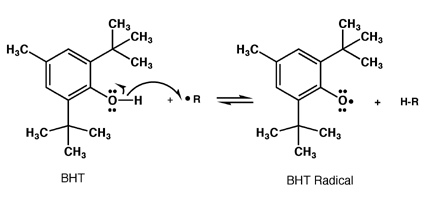
Oxidation can produce free radical species that can be incredibly destructive to surrounding chemicals. Antioxidants aim to reduce free radicals by acting as an electron donor to the radical species.
While this does ultimately end up with a radical antioxidant, the structure is resonance stabilised and is thus significantly less reactive.
Because resonance is such an important component, antioxidants are quite often phenolic structures, such as BHT:
BHa and BHT has been added to cereal to prevent the oils form going rancid, and since their structure is thermally stable, it can e added to biscuits for the same reason. They have however been implicated as carcinogens and are progressively being banned in different countries.
Other antioxidants are ascorbic acid (vitamin C) which is relatively non toxic and gallates, though they’re not permitted in baby food.
BHA and BHT can be extracted using steam distillation or solvent extraction and can be easily quantified using UV-Vis spectrometry as \(\lambda=369\:nm\). To identify them separately, HPLC can be used to first separate them.