Water Content and Drying¶
Definition
Nutrients are compounds in foods that are essential to life and health. They provide us with energy, the building blocks for repair and growth, and substances necessary to regulate chemical processes.
We can classify nutrients into two primary categories:
- Macronutrients
- Proteins
- Carbohydrates
- Dietary fibre
- Alcohol
- Lipids
- Water
- Micronutrients
- Minerals
- Trce elements
- Vitamins
- Fat soluble
- Water soluble
Water¶
All food contains water in some form. This can be measured by looking at water activity (\(a_w\)).
\(a_w\) refers to water that is not bound to food molecules and is a ration of the vapour pressure of the food (\(P\)) to the vapour pressure of pure water (\(P_0\)). as such it ranges from 0 to 1, with most foods having a water activity of \(>0.95\)
Desiccator Jar Method¶
The food is put in a sealed vessel and the air above the sample is measured for humidity. This is then compared with the humidity above a sample of pure water.
Safety and Quality¶
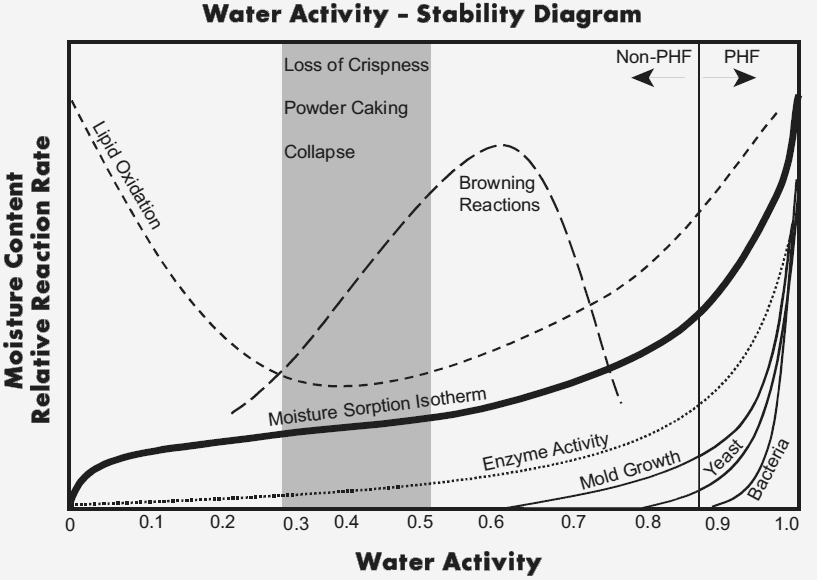
Different values of \(a_w\) will have inherently different chemistry and stability, drastically changing the final product.
Potentially Hazardous Foods (PHF) will typically require refrigeration. in the case of UHF products, they will require refrigeration after opening.
There are a few important numbers to consider:
- Bacteria will not at \(a_w<0.9\)
- Mould will not grow at \(a_w<0.7\)
- No microorganisms will grow at \(a_w<0.6\)
Desiccation (Drying)¶
Drying is a method of food preservation as it can prevent all microbial growth if below a certain vlaue and is often used in compination with salting and smoking. The end goal of desiccation is typically a hydration level of \(3-10\%\) and can be achieved through multiple different methods, such as:
- Sun drying (though contamination is a point of concern)
- Oven drying (at a low temperature)
- Dehydration
- Spray drying
- Microwave-vacuum drying
- Drum drying (spinning the food product at a low temperature)
- Freeze drying (cools the food rapidly then sublimates the water out)
It is worth noting though that the products at that low of a water content are prone to lipid oxidation and Maillard based discolouration
Desiccators¶
Desiccators are vessels with air-tight doors/lids that allow the sample to be isolated from the environment. Without a desiccant, they can be used to measure water sorption (how much water is taken in by the product with respect to, the relative humidity (RH), or \(a_w\), at a constant temperature). Any shelves within the cabinet will have holes in it, so that the air can circulate.
The containers can also be used with a desiccant, such as silica gel, sometimes with anhydrous \(\ce{CoCl2}\) which will turn blue when hydrated to act as an indicator of hydration (\(\color{blue}\ce{CoCl2}\ce{ ->[2H2O] }\color{purple}{\ce{CoCl2.2H2O}}\ce{ ->[4H2O] }\color{magenta}{\ce{[Co(H2O)6]Cl}}\)). This allows them to be used for storage purposes to either reduce the amount of water in a stored product, or to prevent it form absorbing water from the environment, if it’s hygroscopic.
Further variations on the desiccators exist, such as ones with brown glass to store light-sensitive materials and autoclavable desiccators.
Vacuum Desiccators¶
Have a constant vacuum to try decrease the vapour pressure, so that water will always favourable vaporise. It’s important to note that these will also pull gasses out of solution and that any chemicals in the cabinet must be safe to use with the cabinet.
Water Content Determination Methods¶
Loss on Drying (LOD) Method¶
This method determines the water content of the product by weighing it before and after drying. As it’s a primary method of measurement, no calibration is needed, though it makes a big assumption, that all of the mass lost is from water loss. It is also a destructive method and is best suited to fluid/viscous liquid samples.
Distillation Method ¶
Distillation can be used to determine the water content of a sample however a few considerations need to be made
-
The food product must be surrounded with a solvent that must:
- Be water immiscible (no azeotropes)
- Have a higher boiling point than water (so it distills of after the water
- Have a lower density than the water (so that if any does distil over it can be separated out)
The method uses the Dean-Stark apparatus, which collects all of the distillate in a graduated pipette.
Unfortunately, this method is not particularly accurate, as full evaporation is incredibly difficult, though it is useful for foods with low moisture contents. It also will distil over any water soluble compounds within the sample, which may skew the results.
Karl Fischer (KF) Titration Method¶
The method works by titrating a sample with the Karl-Fischer solution, which quantitatively utilises iodine. The iodine reacts with the water, so the titration will continue util excess iodine is present.
This method is specific for water and is very accurate to be used as a test method, however it must be adapted for each specific sample, as it’s not generalisable.