Carbohydrates¶
For carbohydrate structure, see biochem notes attached
The summary of prior knowledge¶
- Carbohydrates can be simple or complex, with simple sugars being monomers and complex having two or more subunits
-
The naming is as follows:
- \(1\) subunit = monosaccharide (glucose/fructose)
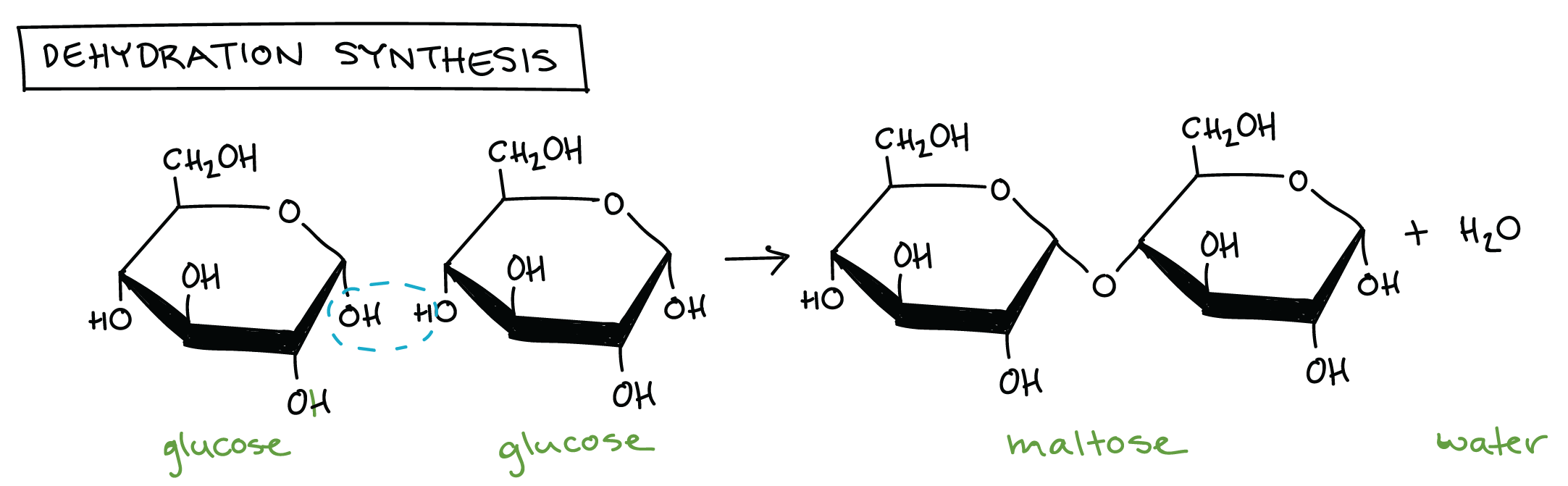
- \(2\) subunits = disaccharide (sucrose/maltose)
- \(3-10\) subunits = oligosaccharide (inulin)
- \(>10\) subunits = polysaccharide (amylose, cellulose)
Monosaccharides¶
Monosaccharides are linear, chains with a ketone or aldehyde and a hydroxyl group off every other carbon
-
They are named as (aldo/keto)(chain length)(ose)
- e.g. aldohexose, ketopentose
- They can be designated L or D chirality based on their interaction with polarised light
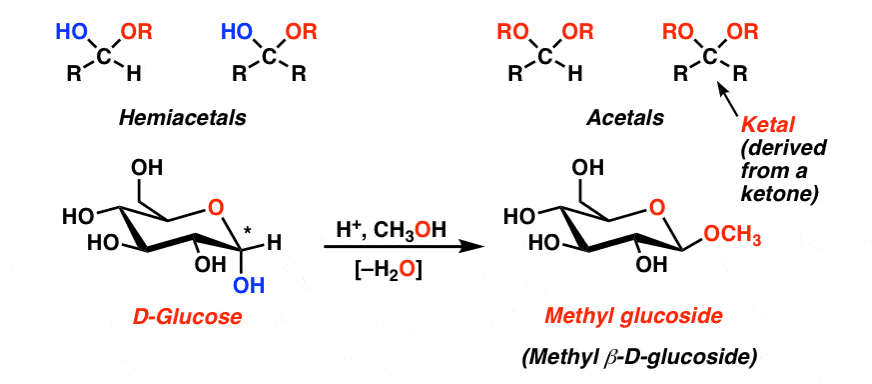
- They will convert between linear and cyclic forms though the formation of a hemiacetal (aldose) or an acetal (ketose)
- Pyranose (pyran base) and Furanose (furan base) sugars are typically stable, however anything smaller than four carbons and there is too much strain.
- The carbon that is now boned to two oxygen molecules is called the anomeric carbon
Disaccharides¶
- All hydroxyl groups on the monosaccharide are capable of undergoing dehydration, however when this occurs on the anomeric carbon, it is unable to linearise.
- All glycosidic bonds require ate least one acetal or hemiacetal, so it’s not possible to get a disaccharide in which both monomers can linearise, but it is common to have a disaccharide in which one end will.
- If a linear sugar is an aldose it can naturally be oxidised to a carboxylic acid, however ketoses require tautomerisation to be able to be reduced.
Linkages¶
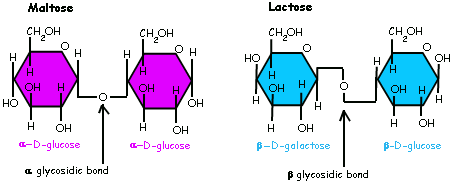
- Are named after the carbons from each subunit and the chirality of those carbons
- E.g. Lactose - \(\beta(1\to4)\)
-
They can also be linked between different chiralities
- e.g. sucrose - \(\alpha,\beta(1\to2)\)
Oligosaccharides¶
- Can be digestible or non-digestible
- All are less sweet than sucrose and trend follows that sweetness is inversely proportional to molecular size
Maltooligosaccharides - Digestible¶
- Are oligosaccharides made up of \(\alpha(1\to4)\) glycosidic linkages between glucose monomers
- Mixtures of these can be used as food additives to serve as sweeteners, and gelling agents
Dietary fiber - non-digestible¶
- pass undigested by the intestine, into the colon where they are fermented by bacteria
- A Prebiotic is a compound that selectively is undigestible by the host and feeds the microorganisms, providing a health benefit
- This is not the same as probiotics which are foods that are enhanced with microorganisms to provide a health benefit
Polysaccharides¶
- Long chains of interlinked monosaccharide monomers
- Naturally occurring polysaccharides tend to be made of glucose monomers
- Cellulose - \(\beta(1\to4)\) - Indigestible plant fibres. Typically structural in nature
- Amylose - \(\alpha(1\to4)\) - Digestible starch fibres. Made of a single long stand that coils when dissolved in water (facilitates the iodine test)
- Amylopectin - \(\alpha(1\to4)\) with \(\alpha(1\to6)\) side chains
- Digestible starch is a mix of 15-30% amylose and 70-85% amylopectin
- Glycogen is stored in the liver and skeletal muscles and is made of amylopectin surrounding a glycogenin protein
- Requires the enzyme glucose-6-phosphatase to digest it
Non-Digestible polysaccharides¶
- Are resistant to enzymatic hydrolysis in the human digestive system. We don’t have the right enzymes to be able to digest it
- Pectins as structural hetero-polysaccharides that consist of linear chains of \(\alpha(1\to4)-D\) galacturonic acid
- The side chains are composed of neutral sugars (10-15%)
- The carboxylic acid groups on the side of the galacturonic acid can be esterified and is quantified as the degree of esterification (DE)
- pectin can be classified as High MethoxyPectin (HMP) which has a DE >50% or Low MethoxyPectin (LMP) with a DE <50%
Carbohydrate Analysis¶
Carbohydrates have a few different measures of quantification, the carbohydrates can either be quantified as :
- Total Carbohydrates - including fibre
- Available carbohydrates - excluding fibre
Both of these measures can be calculated by:
- Difference - what’s left over after you quantify everything else
- Direct analysis - how much of the carbohydrate itself you measure
Methodology¶
There are a few methods that can be used to determine the concentration of carbohydrates:
Refractometry¶
The concentration of a solute within a solution will change its refractive index and thus it is possible to determine the concentration by the refractive index of the solution.
A unit of Brix (Bx) is used to represent 1% of sucrose in solution (\(1\:Bx=1g/100g\) sucrose)
Copper reduction tests (Benedict’s/Fehling’s)¶
Test for the presence of reducing sugars in the sample.
- The sugar in an alkaline environment is converted to an enediol (enol with hydroxyl groups on both sides of the double bond)
- The enediol is then reduced by \(\ce{Cu^{2+}}\) to have two carbonyl groups, producing \(\ce{Cu+}\)
- The copper then forms a red ppt with any \(\ce{OH-}\) (\(\ce{Cu+ + OH- -> CuOH -> Cu2O}\))
Spectroscopy¶
There are two primary methods, the anthrone and phenol/sulphuric acid methods however little is said beyond that:
- Anthrone method can’t be used for milks containing galactose-rich oligosaccharides
- Phenol/sulphuric acid method can’r mrasure amino sugars
Another method uses dinitrosalicylic acid to oxidise the reducing sugars, in turn producing a quantifiable colour change (\(\lambda = 540\:nm\)) that can be measured spectroscopically. This has the caveat that only reducing sugars will be quantified, so it is important to hydrolyse the sample and use strategic methodology to quantify only the carbohydrates that are required.