MS Recap¶
For CHE20006 MS notes see pdf attached
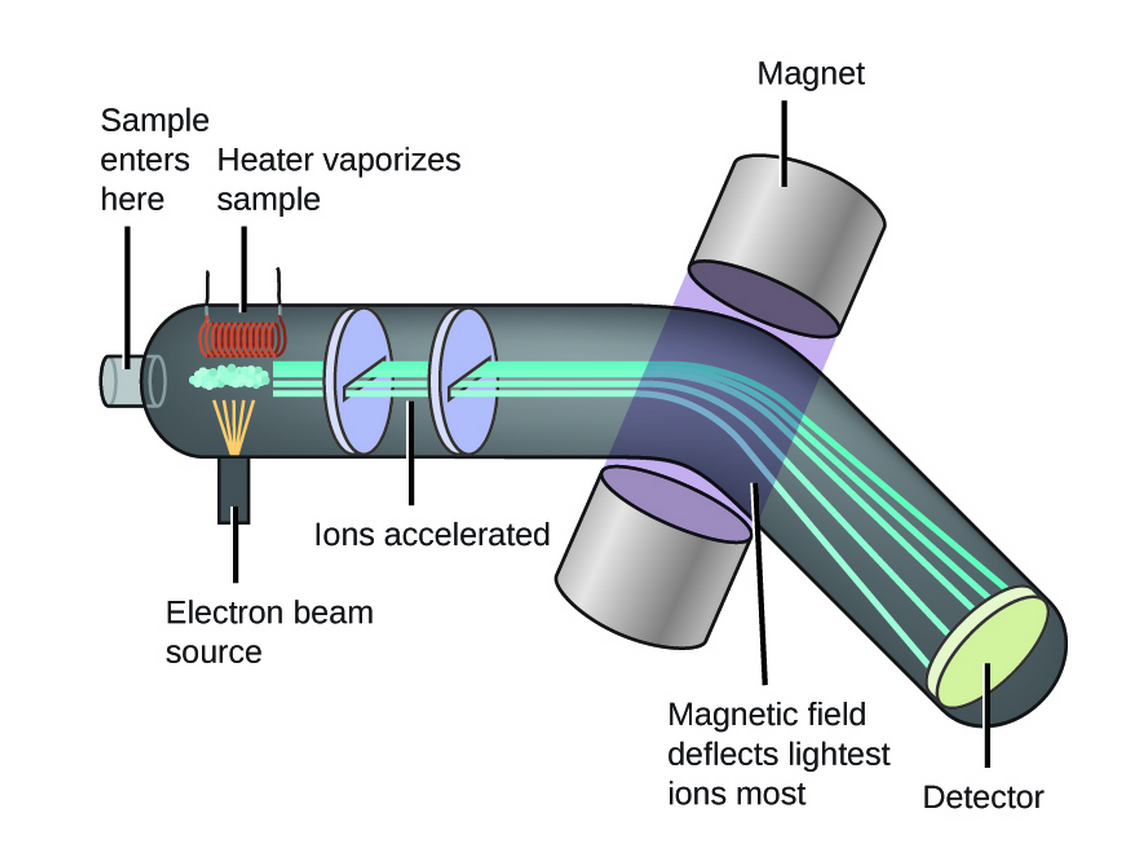
Mass spectrometry is in it’s most simple, the (destructive) ionisation of a sample, followed by the separation of the ion fragments by their mass to charge ratio (m/z).
Terminology
- Molecular ion (\(M^+\)) - The ion obtained by the loss of a single electron from the wole molecule
- Base peak - The most intense peak in the spectrum, assigned 100% intesity
- Radical Cation - a positive charged species with an odd number of electrons
- Fragment Ions - The smaller ions that form from the further fragmentation of the molecule
The ions are formed through multiple means (see CHE20006 notes), however this unit will cover only Electron Ionisation (EI).
In this method, the samples are simply heated to a point at which they vaporise and are bombarded with electrons. While most of the electrons will not interact with the molecule, every now and then an electron will knock into another, displacing it from the molecule, leaving a positive charge. This is then accelerated through series of magnets and is deflected, which using the principles of momentum wlll be variable based on the mass and charge of the molecule
Where:
- \(m=\) mass of the ion
- \(z=\) charge of the ion
- \(B=\) strength of the magnetic field
- \(r=\) radius of the path
- \(V=\) voltage
If enough energy is injected into the system, it’s also possible to break the ion further, creating characteristic fragments for the molecule. Given that most of these species will not be neutral, stable molecules, they may also spontaneously fragment. The distribution of these fragments in the spectrum is therefore an indicator of ionic stability.
Note
Because the process requires the molecule to be in a gaseous phase, it has to be volatile to some extent, but it also needs to be at least somewhat thermally stable (no hyper-strained molecules)
For this reason, the molecular ion is often not seem $$ \begin{align} \ce{M + e- &-> M^{.+} + 2e-}\ \ce{M^{.+} &-> A^{.+} + B}\ \ce{M^{.+} &-> A+ + B^{.}}\ \ce{A+ &-> C+ + D + …} \end{align} $$
Example - Methanol fragmentation
Fragmentation¶
This will often happen in a way that an ion will fragment to produce another ion and a neutral molecule, that will not be detected.
Fragmentation ultimately happens because the fragment (possibly the neutral component) is more stable than the parent ion.
There are a few rules of fragmentation:
- Linear alkyl chains are more stable than branched ones
- The heavier the fragment, the less stable
- Within branched alkyl species, the largest group will be the first to be cleaved
Isotopes¶
Atoms with naturally occurring isotopes will typically have multiple peaks in ratios pertaining to the natural abundance of the isotopes
- Bromine will have two identical peaks two units apart (1:1 \(\ce{^79Br/^81Br}\))
- Chlorine will have two peaks of different abundance, two units apart (3:1 \(\ce{^35Cl/^37Cl}\))
- Carbon might have a much smaller peak (100:1 \(\ce{^12C/^13C}\))
If there are multiple species with multiple naturally occurring isotopes, multiple peaks will form with summing abundance
- E.g. Chlorine - \(\ce{^35Cl2/^35Cl^37Cl/^37Cl2}\)