NMR Recap¶
Continues on from Spectroscopy and Instrumentation¶
NMR Active Nuclei¶
Fo an atom to be NMR active, it needs to have a quantum number \(I\neq0\).
Nuclei with an odd number of protons (atomic number), neutrons (atomic mass) or both with have a nuclear spin.
| # Protons | # Neutrons | \(I\) | Examples |
|---|---|---|---|
| Even | Even | 0 | \(\ce{^{12}C, ^{16}O, ^{32}S}\) |
| Odd | Even | \(1/2\) | \(\ce{^{1}H, ^{19}F, ^{31}P}\) |
| Odd | Even | \(3/2\) | \(\ce{^{11}B, ^{35}Cl, ^{79}Br}\) |
| Even | Odd | \(1/2\) | \(\ce{^{13}C}\) |
| Even | Odd | \(3/2\) | \(\ce{^{127}I}\) |
| Even | Odd | \(5/2\) | \(\ce{^{17}O}\) |
| Odd | Odd | 1 | \(\ce{^{2}H, ^{14}N}\) |
NMR needs these isotopes to be relatively high in concentration, or it needs to sample for an incredibly long period of time.
Chemical Shift¶
Occurs as the different atoms will have a different electron density surrounding them. Each additional electron will have its own associate magnetic field and so as electrons withdrawn or donated, the atom will have a slightly larger or smaller response to the magnetic field. This can happen for many reasons, including intramolecular h-bonding and hybridisation state.
The chemical shift is measured as a difference from the \(0\:ppm\) standard reference (TMS for \(\hnmr\) and \(\cnmr\) )
The values themselves are scaled up by \(\e{6}\), as the shifts are realistically incredibly small.
Shift Tables¶
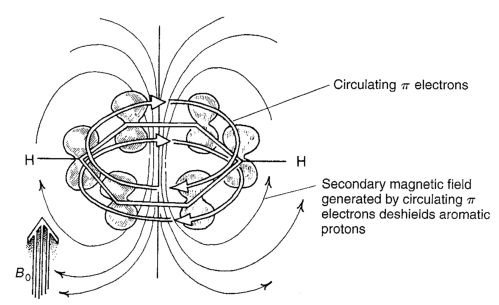
\(\pi\) Induced Magnetic Fields¶
The \(\pi\) bond’s natural ability to conjugate and have free moving electrons, allows the to create an equivalent magnetic field that will reinforce the magnetic field of the NMR spectrometer. This causes a downfield shift for any aromatic or pi bonded carbon atoms.
Splitting¶
Covered in Spectroscopy and Instrumentation, though it’s worth noting that most \(\cnmr\) spectra are decoupled from protons, so you likely won’t see splitting in a \(\cnmr\) spectra.
Integration¶
Covered in Spectroscopy and Instrumentation, though again, \(\cnmr\) spectra are not so easily integrated. This is because the \(\ce{^13C}\) nuclei has a much more varied relaxation period


.pdf+-+SumatraPDF_2012-12-20_00-45-54.png)